Precut, prestained tissue segments may be advantageous in keratoconus surgery
Article originally published on Healio, Nov. 16, 2021.

Corneal allogenic intrastromal ring segments have been described by Jacob and colleagues for the treatment of keratoconus.
They consist of semicircular pieces of donor corneal stroma that may be surgically implanted to flatten, stiffen and stabilize the shape of the recipient cornea.
Early corneal allogenic intrastromal ring segments (CAIRS) protocols used donor segments prepared immediately before implantation, at the time of the surgery. However, because this process involves multiple steps, it may add significantly to the overall procedure time. In addition, any problems encountered during segment creation could jeopardize the ability to complete the operation. Therefore, it may be advantageous to prepare the donor material in advance.
Similar considerations have inspired the recent utilization of so-called “patient-ready” tissue for Descemet’s membrane endothelial keratoplasty, ie, donor endothelium that has been prestripped, prestained and preloaded. Since this concept was introduced in 2016, studies have demonstrated robust stain retention and tissue viability, with surgical outcomes comparable to those in which the tissue is prepared intraoperatively. Patient-ready tissue may also facilitate the uptake of DMEK by novel surgeons by diminishing the anxiety and risks associated with graft preparation.
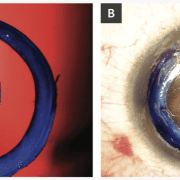
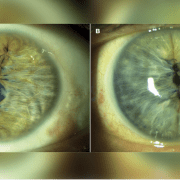
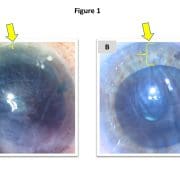
To date, we have performed CAIRS in 16 eyes of 15 patients with keratoconus using precut, prestained tissue. These patient-ready segments are prepared within an eye bank setting 48 to 72 hours before surgery. A donor corneoscleral button is debrided of epithelium and endothelium using a surgical sponge and then mounted on a Teflon cutting block. A dedicated CAIRS trephine (Jacob CAIRS trephine, Madhu Instruments) is used to create a circular tissue ring that is bisected via surgical knife into two semicircular segments. The option also exists to supply it as a full ring to allow customization of arc length by the surgeon. Both segments are submerged in 0.06% trypan blue (VisionBlue, DORC International) for 3 minutes until a dark blue tissue stain is obtained and then replaced in storage solution and photographed (Figure 1a). At the time of surgery, the segments are removed from storage, irrigated with saline, dehydrated atop a surgical sponge and implanted (Figure 1b).
Since transitioning to patient-ready CAIRS, no additional intraoperative or postoperative complications have been experienced. As with prestripped and prestained DMEK grafts, the tissue retains its blue stain throughout the duration of the procedure but fades completely by several days postoperatively.
Advantages of patient-ready CAIRS may include reduced surgical time, flattened surgical learning curve and increased consistency of product. As a result, this may become a preferred option for tissue preparation, especially for surgeons starting with the technique.
References:
- Jacob S, et al. J Refract Surg. 2018;doi:10.3928/1081597X-20180223-01.
- Parekh M, et al. Am J Ophthalmol. 2016;doi:10.1016/j.ajo.2016.03.048.
- Parker JS, et al. J Cataract Refract Surg. 2021;doi:10.1097/j.jcrs.0000000000000316.
- Parker JS, et al. J Cataract Refract Surg. 2021;doi:10.1097/j.jcrs.0000000000000582.
- Potts LB, et al. Cornea. 2020;doi:10.1097/ICO.0000000000002400.
- Zafar S, et al. Clin Ophthalmol. 2019;doi:10.2147/OPTH.S212871.
For more information:
Hudson Tate, David Blackburn and Jack S. Parker, MD, PhD, can be reached at Parker Cornea, 700 18th St. South, Suite 503, Birmingham, AL 35233; emails: hdtate@crimson.ua.edu; davidblackb60@gmail.com; jack.parker@gmail.com.